Проблемы здоровья и качества жизни женщин в пери- и постменопаузе в последние десятилетия приобретают особое приоритетное значение в связи с увеличением продолжительности жизни во всем мире. За период с 1950 по 2017 гг. средняя продолжительность жизни женщин увеличилась более чем на 22 года: с 52,9 года в 1950 г. до 75,6 года в 2017 г., а в настоящее время этот показатель приближается к 80 годам [1]. Согласно статистическим прогнозам, к 2050 г. 30% мирового населения достигнут возраста 65 лет и более [2].
Долголетие сопровождается увеличением количества лет, прожитых с хроническими болезнями и инвалидностью. У женщин в постменопаузе значимо повышается риск развития ожирения, инсулинорезистентности, сахарного диабета 2 типа (СД2), когнитивных нарушений, деменции, остеопороза. Наступление менопаузы четко ассоциировано с увеличением риска сердечно-сосудистых заболеваний (ССЗ). Ишемическая болезнь сердца, сердечная недостаточность и инсульт являются ведущими причинами смерти женщин в возрасте 60 лет и старше во всем мире [3]. Понимание факторов, которые поддерживают здоровье и активность женщин с возрастом, жизненно важно, учитывая, что средняя продолжительность жизни девочки, родившейся в 2030 г., вероятно, превысит 90 лет [4]. Считается, что половые гормоны играют решающую роль в развитии болезней старения, при этом у женщин основное внимание уделяется эстрогенам. Значение андрогенов в патогенезе ССЗ и других возрастных изменений до недавнего времени оставалось неопределенным, хотя имеющиеся литературные данные свидетельствуют о том, что эндогенный тестостерон оказывает протективные эффекты на здоровье женщин в постменопаузе.
Синтез и физиологические свойства андрогенов в женском организме
По химической структуре андрогены представляют собой 19-углеродные стероидные гормоны, в основе которых лежит скелет молекулы углеводорода сложного строения – андростана. Основным андрогеном как у мужчин, так и у женщин является тестостерон, который вырабатывается в гонадах или в периферических тканях из прогормонов: дегидроэпиандростерона (ДГЭА) и андростендиона [5]. ДГЭА – полифункциональный стероидный гормон, большая часть которого (80%) продуцируется сетчатой зоной надпочечников. В надпочечниках ДГЭА метаболизируется с образованием андростендиола, андростендиона, а также дегидроэпиандростерон-сульфата (ДГЭА-C) [6]. Неактивная форма ДГЭА-C попадает в плазму крови и в периферических тканях с помощью интракринных механизмов метаболизируется с образованием активных форм андрогенов и эстрогенов [6]. В настоящее время идентифицировано более 30 тканеспецифичных генов/ферментов, регулирующих как внутриклеточное образование, так и инактивацию половых стероидов в соответствии с конкретными потребностями органа или ткани [7]. Ферменты глюкуронилтрансферазы и сульфотрансферазы инактивируют внутриклеточно андрогены и эстрогены, таким образом поддерживая уровни активных половых стероидов в сыворотке крови в пределах низких концентраций, что особенно важно для периода постменопаузы [7].
Андростендион – андрогенный стероид, вырабатывается яичниками и корой надпочечников у женщин. Во внегонадных тканях-мишенях, таких как мозг, кости, жировая ткань, кожа, происходит конверсия андростендиона путем ароматизации в эстрон либо под воздействием фермента 17β-гидроксистероиддегидрогеназы – в тестостерон. В свою очередь, тестостерон преобразуется в эстрадиол под влиянием ароматазы или в дигидротестостерон (ДГТ) при участии 5α-редуктазы [6, 7]. Чувствительность тканей к андрогенам варьирует в зависимости от активности тканевых рецепторов к андрогенам, а также от количества и активности ферментов 5α-редуктазы и ароматазы, которые могут значительно различаться у разных женщин (рис. 1).
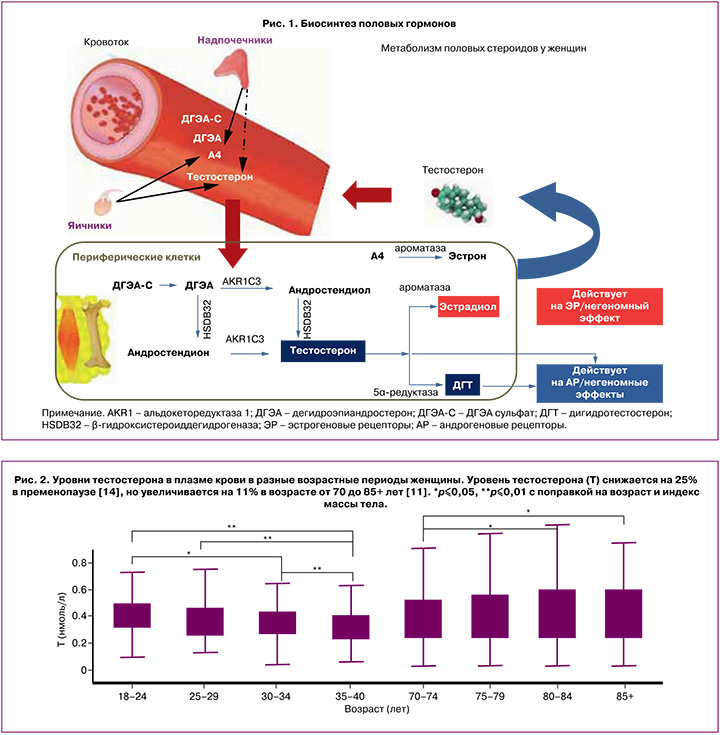
Примерно две трети тестостерона в крови связано с глобулином, связывающим половые гормоны (ГСПГ), почти треть слабо связана с альбумином и другими белками плазмы, и лишь незначительное количество циркулирует в свободном состоянии [8]. Показано, что несвязанный тестостерон является наиболее легко разлагаемой фракцией и, следовательно, наименее метаболически активной [9]. Кроме того, измерение уровня несвязанного тестостерона осложняется низкой точностью и специфичностью большинства методов, а формулы, используемые для расчета свободной фракции, недостаточно достоверны и дают противоречивые результаты [8]. В связи с этим международная комиссия пришла к выводу, что определение уровня общего тестостерона остается наиболее оптимальным методом для оценки функциональной активности андрогенов у женщин [10].
Стандартом для измерения уровней тестостерона и ДГТ является жидкостная хроматография с тандемной масс-спектрометрией (LCMS, ЖХ-ТМС). В ряде исследований с использованием ЖХ-ТМС показано, что концентрации тестостерона, андростендиона и ДГТ в плазме крови снижаются к периоду менопаузы на 25–35%, а ДГЭА – на 60% [6, 11]. В постменопаузе тека-клетки яичников продолжают вырабатывать тестостерон в ответ на повышение уровня гонадотропинов. Уровень тестостерона у женщин достигает минимума в 60–65 лет, а затем имеет тенденцию к повышению. Показано, что концентрация тестостерона у женщин в возрасте 70 лет и старше аналогична концентрации, наблюдаемой у женщин в пременопаузе (рис. 2) [11–13]. Возможно, это связано со снижением активности ароматазы, но точные причины данного феномена пока неизвестны.
Ранее проведенные исследования определили взаимосвязь дисбаланса эстрогенов и андрогенов у женщин в постменопаузе, а также повышение уровня тестостерона в возрасте 70–80 лет с повышением риска метаболических нарушений и ССЗ [15], но обновленные результаты механистических и клинических обследований не поддерживают данную точку зрения.
Влияние эндогенных андрогенов на сердечно-сосудистую систему и метаболические параметры (экспериментальные и клинические данные)
Дисфункция эндотелия сосудов играет ведущую роль в патогенезе гипертонической болезни и ишемической болезни сердца [16]. В механистическом исследовании тестостерон увеличивал выработку оксида азота в эндотелиальных и гладкомышечных клетках аорты крыс [17]. Эти эффекты были заблокированы финастеридом, антагонистом рецепторов андрогенов [17]. Это указывает на опосредованный рецепторами андрогенов эффект тестостерона на выработку или высвобождение эндотелием оксида азота. Влияние тестостерона на ишемию миокарда изучалось на моделях мышей. Кардиомиоциты обладают ароматазой и могут вырабатывать эстрадиол из тестостерона и андростендиона [18]. Мыши с нокаутом ароматазы (ArKO) не могут синтезировать эстрогены и, подобно другим грызунам, не вырабатывают ДГЭА [19]. Поскольку они не могут преобразовывать тестостерон в эстрадиол, у мышей ArKO концентрация тестостерона выше, чем у мышей контрольной группы. Несмотря на полное истощение эстрогенов, во время реперфузии сердца после эпизода ишемии мыши ArKO демонстрировали более высокие систолические показатели, меньшую диастолическую дисфункцию и меньший выброс лактатдегидрогеназы, что свидетельствует о менее выраженном повреждении сердечной мышцы по сравнению с группой контроля [19].
В исследовании Montalcini T. et al. оценена взаимосвязь между уровнем эндогенного тестостерона и функцией эндотелия сосудов у 60 женщин в постменопаузе со средним возрастом 56,9 года и длительностью постменопаузы в среднем 9,4 года. После поправки на искажающие факторы установлено, что уровень тестостерона в плазме крови положительно коррелировал с опосредованной кровотоком дилатацией плечевой артерии (β=0,277, P=0,03) [20]. В более позднем исследовании Rech C.M. et al. приняли участие 36 женщин с двусторонней овариоэктомией (группа O) и 45 женщин контрольной группы (группа C) со средней продолжительностью постменопаузы 10 лет, принимающих менопаузальную гормонотерапию. Уровни эстрадиола, тиреотропного гормона, биохимические показатели и состав тела не различались между группами, а уровень тестостерона в плазме крови был значимо ниже в группе О, чем в группе С (11,0 против 23,0 нг/дл, P=0,001). Показатели тестостерона прямо коррелировали с кровотоком в плечевой артерии и были статистически значимо ниже в группе О по сравнению с контролем как на исходном уровне (1,57 против 2,19, Р=0,036), так и после эндотелий-зависимого расширения, опосредованного кровотоком (3,44 против 4,3, Р=0,031), и после приема нитроглицерина (эндотелий-независимое расширение, 1,39 против 1,76, Р=0,025) [21].
Переход к менопаузе связан с неблагоприятным воздействием на липидный профиль, сопровождающимся повышением уровня общего холестерина (ХC), холестерина липопротеинов низкой плотности (ЛПНП), триглицеридов (Tц) и липопротеина (a), а иногда и снижением уровня липопротеинов высокой плотности (ЛПВП) [22]. Хорошо известно, что неблагоприятный липидный профиль играет важную роль в развитии и прогрессировании ССЗ. Исследования взаимосвязи между уровнем эндогенного тестостерона и липидами крови демонстрируют противоречивые результаты. В немецком исследовании проанализированы данные 2560 женщин из двух когорт, участвовавших в исследовании «Здоровье в Померании». Показана прямая корреляция между уровнем общего тестостерона и ХС (β-коэффициент: 0,03; 95% ДИ: 0,01; 0,05), а также общего тестостерона и ЛПНП (β-коэффициент: 0,02; 95% ДИ: 0,01; 0,05) [23]. С другой стороны, в когортном исследовании Bell R. et al. с участием 587 женщин в возрасте от 18 до 75 лет установлено, что эндогенный тестостерон и его предшественники не являются значимыми независимыми факторами, влияющими на уровень высокочувствительного С-реактивного белка (СРБ) или липидов плазмы крови. Статистически значимые изменения наблюдались при добавлении ГСПГ в модели для СРБ и ЛПВП как для женщин в пременопаузе, так и для женщин в постменопаузе (P≤0,01 и <0,001 соответственно) [24]. В многонациональном когортном исследовании (США, Канада, Австралия, Великобритания и Швеция) приняли участие 624 женщины в постменопаузе от 40 до 60 лет, не принимающие менопаузальную гормонотерапию или препараты для снижения уровня липидов. Взаимосвязь между уровнями тестостерона, ДГТ, эстрона, эстрадиола, ГСПГ и каждой липидной переменной изучались с помощью многофакторной линейной регрессии. Результаты показали, что ни один из измеренных половых стероидов не вносил независимого вклада в многофакторные модели для ХС, ЛПВП, ЛПНП или Тц. В большей степени на липидный профиль влияли возраст, раса, артериальное давление (АД) и хирургическая менопауза [25].
В том же многонациональном исследовании у 763 женщин в постменопаузе оценена взаимосвязь между логарифмически преобразованным уровнем С-пептида и резистентностью к инсулину (HOMA-IR) с учетом возраста, расы, индекса массы тела (ИМТ), АД и уровнями эстрадиола, эстрона, тестостерона и ДГТ. Выявлено, что ни один из половых стероидов не оказывал независимого влияния на индекс HOMA-IR, который в большей степени коррелировал с ИМТ, уровнем С-пептида, систолическим АД и хирургической менопаузой (r(2)=0,3152, P=0,03) [26]. В ходе Роттердамского исследования проанализированы данные 3117 женщин в постменопаузе с целью определения влияния эстрадиола, тестостерона, свободного тестостерона и ГСПГ на риск развития СД2. В ходе наблюдения, которое в среднем составило 11,1 лет, выявлено 384 случая СД2. Авторы не обнаружили связи между уровнем общего тестостерона, измеренным методом ЖХ-ТМС, или биодоступным тестостероном и развитием СД2. Уровень ГСПГ был обратно пропорционален риску СД2 (ОР 0,56 [95% ДИ 0,41–0,77], P<0,001), в то время как уровень общего эстрадиола прямо коррелировал с повышенным риском СД2 (ОР 1,39 [95% ДИ 1,004–1,93], P=0,07) [27]. Кроме того, те же авторы в метаанализе 13 проспективных исследований с участием более 1912 пациентов с впервые выявленным СД2 подтвердили, что низкий уровень ГСПГ и высокий уровень эстрадиола были связаны с повышенным риском СД2, в то время как для других гормонов такой связи обнаружено не было. Таким образом, был сделан вывод, что низкий уровень ГСПГ и высокий уровень эстрадиола являются независимыми факторами риска развития СД2 у женщин [27].
Старые обсервационные исследования по изучению взаимосвязи частоты ишемических сердечно-сосудистых событий и смертности с уровнем эндогенного тестостерона имели ряд серьезных ограничений: дизайн «случай–контроль», большие интервалы между временем забора крови и сердечно-сосудистыми событиями и использование иммуноанализов, которым не хватает точности для измерения уровня половых стероидов [28–30]. В более позднем крупном популяционном исследовании KORA Schederecker Е. et al. изучали влияние эндогенных андрогенов и ГСПГ на смертность от ССЗ и всех причин у 1006 мужчин и 709 женщин в возрасте от 45 до 82 лет. В ходе наблюдения (IQR: 8,2–9,2 года) из 709 участниц исследования 70 (9,9%) умерли: 35,7% – от ССЗ, 37,1% – от рака и 27,1% – от других заболеваний. У женщин в многофакторных скорректированных моделях уровень ГСПГ положительно коррелировал со смертностью от всех причин (ОР: 1,54, 95% ДИ: 1,16–2,04). При этом уровень андрогенов (тестостерона, ДГТ), измеренный с помощью ЖХ-ТМС, не был связан с повышением риска смертности от ССЗ и всех причин (P<0,05) [31].
В проспективном когортном исследовании Sievers C. et al. с включением 2914 пациенток в возрасте от 18 до 75 лет показано, что женщины с уровнем общего тестостерона в нижнем квартиле Q1 имели более высокий риск смерти от любой причины или развития сердечно-сосудистых событий в течение периода наблюдения, по сравнению с пациентами с более высокими показателями общего тестостерона (квартили Q2–Q5), в скорректированных моделях регрессии Кокса (смертность от всех причин: Q2–Q5 по сравнению с Q1: ОР 0,62, 95% ДИ 0,42–0,939; сердечно-сосудистые события: Q2–Q5 по сравнению с Q1: ОР 0,68, 95% ДИ 0,48–0,97) [32].
В исследовании SHOW «Половые гормоны у пожилых женщин» изучалась взаимосвязь между концентрацией половых стероидов в крови, измеренной методом ЖХ-ТМС, и риском серьезных сердечно-сосудистых осложнений (ССО). Это проспективное когортное австралийское исследование в рамках лонгитюдного проекта ASPREE, в которое были включены 5535 женщин старше 70 лет (средний возраст 74 года) без когнитивных нарушений, предшествующих ССЗ с ожидаемой продолжительностью жизни не менее 5 лет, не получающих менопаузальную гормонотерапию. В течение периода наблюдения (в среднем 4,6 года) у 144 (2,6%) женщин впервые развился инфаркт миокарда или инсульт (частота 5,9 на 1000 человеко-лет), умерли 200 женщин (7,9 на 1000 человеко-лет). В полностью скорректированных моделях более высокая концентрация тестостерона в плазме крови была связана с более низкой частотой ССО (4-й квартиль по сравнению с 1-м квартилем: коэффициент риска 0,57 [95% ДИ 0,36–0,91]; p=0,02). Аналогичная обратная зависимость наблюдалась и для ДГЭА (4-й квартиль по сравнению с 1-м квартилем: 0,61 [0,38–0,97]; p=0,04). Для эстрогена более низкий риск сердечно-сосудистых событий наблюдался только при концентрации во 2-м квартиле по сравнению с 1-м квартилем (0,55 [0,33–0,92]; p=0,02). В скорректированных моделях не выявлено связи между уровнем ГСПГ и ССО, а также между уровнем любого гормона или ГСПГ и смертностью от иных причин [33]. В рамках того же исследования Davis S.R. et al. оценили взаимосвязь между половыми стероидами и липидами плазмы крови у 3231 австралийской женщины в возрасте старше 70 лет без серьезных ССЗ в анамнезе. С использованием многолинейной регрессии установлено, что по сравнению с концентрацией в самом низком квартиле (Q1) уровень тестостерона в самых высоких квартилях (Q3 и Q4) был положительно связан с уровнем ЛПВП (p=0,002 и p<0,001 соответственно) и обратно пропорционален уровню Тц (p=0,024, p=0,003 и p<0,001 соответственно). Для ДГЭА концентрация в самом высоком квартиле Q4 была положительно связана с уровнем ХС ЛПВП (p=0,024). Данные результаты свидетельствуют о потенциально протективном влиянии тестостерона и ДГЭА на липидный профиль и сердечно-сосудистую систему у пожилых женщин [34].
Влияние эндогенных андрогенов на минеральную плотность костной ткани и мышечную силу у женщин в пери- и постменопаузе
Остеопороз – это заболевание, при котором повышается хрупкость костей из-за снижения их плотности и разрушения костной микроструктуры. Остеопороз – одна из основных причин переломов позвоночника и шейки бедра у женщин среднего и пожилого возраста, которая серьезно влияет на качество жизни, является причиной инвалидизации и увеличивает социально-экономическое бремя [35]. Ряд исследований свидетельствует о том, что не только дефицит эстрогенов, но и снижение уровня эндогенных андрогенов тесно связано с нарушением баланса процесса образования и резорбции костной ткани у женщин в постменопаузе.
В поперечном исследовании в рамках Национального обследования состояния здоровья и питания в США NHANES оценена взаимосвязь между уровнем тестостерона в плазме крови и минеральной плотностью костной ткани (МПКТ) у женщин в возрасте 40–60 лет (N=2198). Результаты показали, что уровень тестостерона положительно коррелировал с МПКТ поясничного отдела позвоночника после учета всех сопутствующих факторов (β=1,12, 95% ДИ 0,31–1,93) вне зависимости от приема менопаузальной гормонотерапии и комбинированных оральных контрацептивов [36].
Ghebre М.А. et al. определяли зависимость между уровнем ДГЭА-С в сыворотке крови и изменением МПКТ в шейке бедренной кости и поясничном отделе позвоночника у 1003 женщин в постменопаузе в возрасте от 45 до 68 лет. Установлено, что повышение уровня ДГЭА-С (на каждый микромоль на литр) было связано с уменьшением потери костной массы в шейке бедра на 0,49% (95% ДИ 0,21–0,71%, P=0,001). Аналогичные, но более слабые результаты были получены для МПКТ в поясничном отделе позвоночника. Результаты свидетельствуют о том, что высокий уровень ДГЭА-С в сыворотке крови связан с меньшей потерей костной массы у женщин в постменопаузе, но эта связь со временем ослабевает [37].
C возрастом происходит постепенное снижение силы и массы скелетных мышц, изменение их состава в связи с жировой инфильтрацией. Это явление известно как саркопения и затрагивает около 30% населения старше 60 лет [38]. Несмотря на то что андрогены являются анаболическими стероидами, в систематическом обзоре 10 обсервационных исследований не было обнаружено связи между уровнем тестостерона в плазме крови и мышечной массой, силой или работоспособностью у женщин в постменопаузе. Однако авторы публикации предупреждают, что данные результаты следует интерпретировать с осторожностью из-за использования иммуноферментного анализа в подавляющем большинстве включенных в обзор исследований и неточности расчетных показателей, а также из-за неустановленной биологической значимости не связанного с белком тестостерона [39].
В когортном исследовании Wang Y. et al. изучена взаимосвязь между концентрацией половых гормонов в плазме крови, измеренных с помощью ЖХ-ТМС, и физической функцией (силой сжатия кисти и самооценкой физической функции, оцениваемой с помощью SF-12v2) у 5447 женщин в постменопаузе старше 70 лет. С поправками на искажающие факторы показано, что концентрация тестостерона и ДГЭА выше нижнего квартиля была связана с меньшим снижением силы хвата (-1,39 vs -1,75 и -1,39 vs -1,82 соответственно, р=0,02) и с меньшей вероятностью снижения физической активности по сравнению с показателями в соответствующем низшем квартиле [40].
В обсервационном исследовании с использованием данных Многонационального исследования атеросклероза приняли участие 682 женщины в постменопаузе в возрасте от 45 до 84 лет. С помощью компьютерной томографии брюшной полости авторы оценили площадь мышц живота (см2) и их радиоплотность (единицы Хаунсфилда) в зависимости от уровня тестостерона (общего и свободного), эстрадиола и ГСПГ в сыворотке крови. С помощью многовариантных моделей линейной регрессии после поправки на искажающие факторы обнаружено, что более высокие уровни эстрадиола и свободного тестостерона положительно коррелировали с общей площадью мышц брюшного пресса (β=1,41, 95% ДИ 0,4; 2,4, р=0,007 и β=18,5, 95% ДИ 4,0; 33,1, р=0,004 соответственно), но не с рентгеноплотностью мышц (р>0,05). И наоборот, повышенный уровень ГСПГ был связан с меньшей общей площадью брюшных мышц и радиоплотностью (β=-2,1, 95% ДИ -3,2; -0,9, p=0,001 и β=-0,32, 95% ДИ -0,6; -0,0, p=0,07 соответственно). Таким образом, отрицательная связь между ГСПГ и составом мышц может указывать на его потенциальную роль в накоплении жировой ткани в скелетных мышцах и развитии саркопенического ожирения [41]
Влияние эндогенных андрогенов на когнитивную функцию у женщин в пери- и постменопаузе
Согласно данным популяционных исследований, распространенность когнитивных нарушений у женщин в постменопаузе достигает 44–60% [42, 43]. Самыми частыми проявлениями нарушения когнитивной функции в этом периоде являются трудности с запоминанием слов и цифр, дефицит внимания, снижение скорости реакции и нарушения вербальной памяти. По сравнению с мужчинами, у женщин того же возраста отмечены более высокий риск развития болезни Альцгеймера и более выраженная динамика когнитивных нарушений по мере прогрессирования заболевания [44, 45]. Во многих исследованиях, в том числе когортных, изучалось влияние эндогенных и экзогенных эстрогенов на здоровье мозга, но при этом очень мало работ оценивало влияние тестостерона и его метаболитов на когнитивные способности и риск развития деменции у женщин в постменопаузе [46–49]. В ряде экспериментальных исследований показано нейропротективное действие тестостерона, которое выражалось в увеличении количества синапсов в гиппокампе, повышении пластичности нейронов и снижении реактивности астроцитов при повреждении мозга у экспериментальных животных [50–52]. Dratva M.A. et al. проанализировали взаимосвязь между уровнем тестостерона и общим когнитивным статусом у 213 женщин и 348 мужчин в возрасте от 55 до 90 лет из Инициативы по нейровизуализации при болезни Альцгеймера (ADNI). 52% всей выборки были носителями APOE-ε4. В клинических диагностических группах (нормальное когнитивное функционирование, умеренные когнитивные нарушения и болезнь Альцгеймера) авторы обнаружили значимую корреляционную зависимость между уровнем тестостерона в плазме крови и APOE-ε4 у женщин, при которой более низкий уровень тестостерона был связан с ухудшением глобального когнитивного функционирования (β=0,14, SE=0,001, p=0,10), скорости обработки информации и вербальной памяти только у носителей APOE-ε4. При этом у мужчин такой зависимости не было выявлено [53].
В рамках когортного австралийского исследования SHOW изучена взаимосвязь между уровнем половых стероидов в крови, измеренной методом ЖХ-ТМС, ГСПГ и когнитивными способностями у 5511 женщин старше 70 лет, не страдающих деменцией и не принимающих гормональные препараты. Регрессионный анализ с включением возраста, ИМТ, образования, курения, употребления алкоголя, условий жизни, диабета, гипертонии, депрессии и нарушений функции почек не обнаружил связи между уровнями эстрона, эстрадиола, тестостерона или ДГЭА и когнитивными функциями. Однако выявлена обратная корреляционная зависимость между концентрацией ГСПГ и скоростью обработки информации у женщин старшей возрастной группы (Q2, β=-0,94, р=0,009; Q3, β=-0,82, р=0,025; Q4, β=-0,95, р=0,013) [54].
В систематическом обзоре 2023 г. с включением 10 исследований оценивалось влияние эндогенного тестостерона на когнитивные функции у женщин в постменопаузе. В восьми исследованиях уровень тестостерона измерялся с помощью иммуноферментного анализа, в одном – с помощью ЖХ-ТМС, еще в одном – методология определения уровня гормонов не указывалась. В совокупности тестировались 11 различных когнитивных функций с помощью 37 различных инструментов. Независимо от дизайна исследования, результаты были противоречивыми и неубедительными: как положительные, так и отрицательные связи были отмечены для каждого из показателей общего когнитивного функционирования, вербальной памяти и уровня тестостерона в плазме крови. Авторы сделали заключение, что полученные результаты следует считать неубедительными из-за неточности измерения уровня тестостерона и методологической неоднородности включенных исследований, и указали на необходимости проведения дальнейших исследований для решения данного вопроса [55].
Влияние менопаузальной гормонотерапии на уровень эндогенных андрогенов
Менопаузальная гормональная терапия является самой эффективной стратегией в коррекции менопаузальных симптомов и профилактике болезней старения у женщин в пери- и постменопаузе. Учитывая положительное влияние эндогенного тестостерона и его метаболитов на сердечно-сосудистую систему, когнитивные функции, костно-мышечную систему, настроение и либидо, особое значение при выборе менопаузальной гормонотерапии приобретает вопрос сохранения физиологического уровня андрогенов. В зависимости от дозы, пути введения и состава, менопаузальная гормональная терапия оказывает различное влияние на уровень эндогенных андрогенов.
Применение пероральной менопаузальной гормонотерапии с эстрадиолом способствует повышению синтеза ГСПГ, основного белка-переносчика стероидных гормонов, за счет эффекта первичного прохождения через печень. При этом гестагенный компонент в составе гормональной терапии в большей степени влияет на уровень эндогенных андрогенов. Важно понимать, что гестагены существенно различаются между собой – их сродство к рецепторам других стероидных гормонов обуславливает дополнительные, иногда нежелательные, фармакологические эффекты (антиандрогенный, глюкокортикоидный и т.д.) [56, 57]. Гестаген в рационально подобранной комбинированной менопаузальной гормонотерапии должен обладать следующими свойствами:
- надежно защищать эндометрий от пролиферативного эффекта эстрадиола;
- не должен снижать эффективность эстрадиола;
- не должен изменять физиологический уровень эндогенных гормонов, в том числе андрогенов;
- быть метаболически нейтральным и обладать высокоселективным действием.
Дидрогестерон, представляя собой высокоселективный нейтральный гестаген, не снижает уровень эндогенных андрогенов и при этом не снижает благоприятные эффекты эстрадиола в отношении сердечно-сосудистой, опорно-двигательной и нервной систем [58–60]. Сравнительное исследование Rizzo M.R. et al. продемонстрировало, что естественный уровень тестостерона сохранялся при применении менопаузальной гормонотерапии с дидрогестероном (0,33±0,1 нг/мл; p>0,05), в отличие от выраженного снижения при применении менопаузальной гормонотерапии с дроспиреноном (0,26±0,2 нг/мл; p<0,01) [61].
Заключение
Таким образом, подчеркивается необходимость персонализированного подхода к выбору лечения с учетом индивидуальных особенностей пациентки. В этом контексте фиксированная комбинация эстрадиола с дидрогестероном (Фемостон) обладает доказанной эффективностью, благоприятным профилем безопасности и переносимости при длительном применении. Важным преимуществом данной комбинации является ее способность поддерживать естественный гормональный баланс без снижения уровня эндогенных андрогенов. Следует помнить, что оптимальный результат лечения достигается только при комплексном подходе, включающем не только правильно подобранную медикаментозную терапию, но и обязательную коррекцию образа жизни, которая значительно усиливает эффективность проводимого лечения. Такой комплексный подход позволяет улучшить качество жизни женщин и обеспечить здоровое активное долголетие.



