Интегральным этапом программы экстракорпорального оплодотворения (ЭКО) является проведение контролируемой овариальной стимуляции.
Овариальная стимуляция — это фармакологическая терапия, направленная на индукцию развития фолликулов. Именно от результата индукции множественного роста фолликулов, а именно качества и количества получаемых ооцитов, в конечном итоге зависит эффективность программы. Общеизвестным является факт наличия прямой корреляции между количеством получаемых ооцитов и частотой наступления беременности. Таким образом, выбор протокола овариальной стимуляции и конкретного препарата может напрямую повлиять на частоту наступления беременности в программах ЭКО.
В программах вспомогательных репродуктивных технологий (ВРТ) пациентке рекомендовано индивидуально назначать протокол овариальной стимуляции с использованием агонистов гонадотропин-рилизинг-гормона (аГнРГ; бусерелин, гозерелин, трипторелин) или антагонистов гонадотропин-рилизинг-гормона (антГнРГ; ганиреликс, цетрореликс) с учетом возраста и овариального резерва пациентки, риска развития синдрома гиперстимуляции яичников (СГЯ) и особенностей предыдущих циклов овариальной стимуляции.
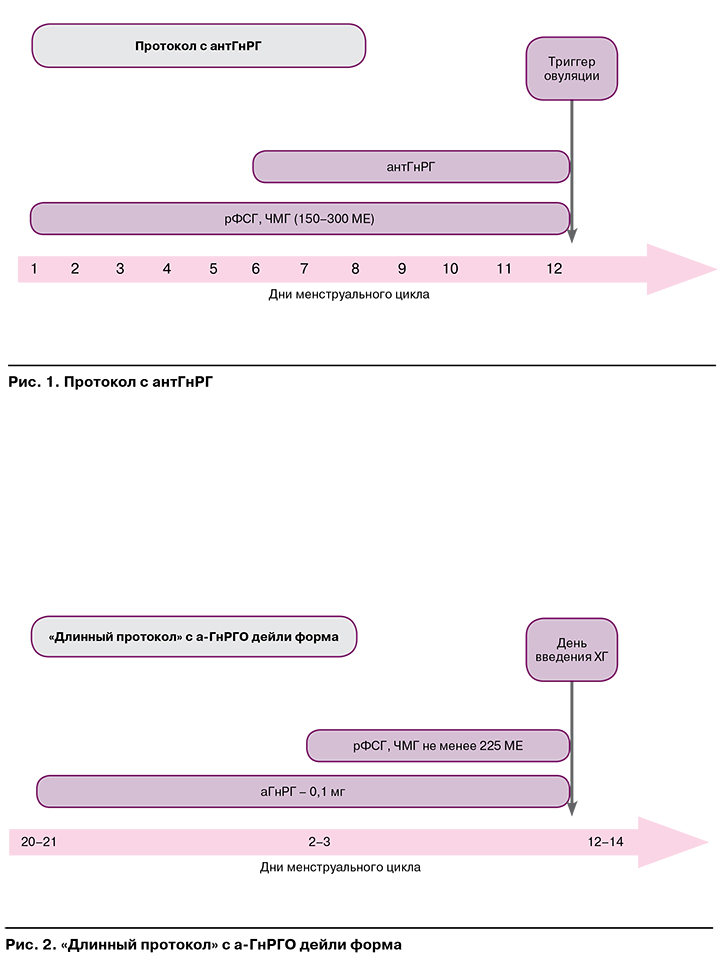
Протокол с антГнРГ рекомендован пациенткам:
- с высоким овариальным резервом;
- синдромом поликистозных яичников;
- дефицитом массы тела;
- нормальным овариальным резервом и первым предстоящим протоколом ЭКО;
- донорам ооцитов;
СГЯ в анамнезе.
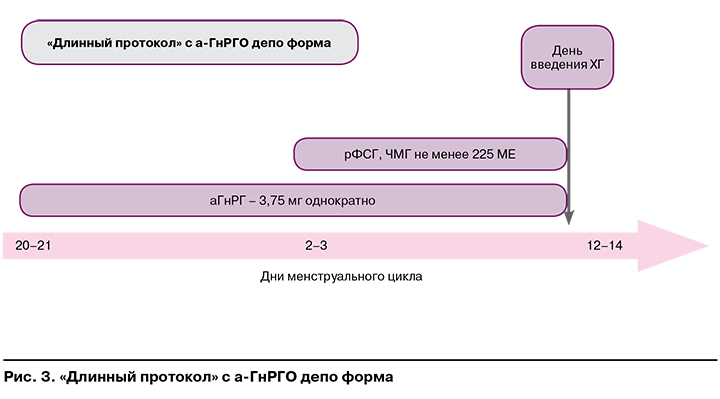
Протоколы с аГнРГ (длинный, короткий, супердлинный) рекомендованы:
- при отсутствии факторов риска развития СГЯ;
- необходимости длительной супрессии гипоталамо-гипофизарно-яичниковой системы при генитальном эндометриозе.
На рисунках 1—3 представлены наиболее часто используемые в рутинной клинической практике подходы к овариальной стимуляции.
Сравнительно недавно исследователи показали возможность индуцировать экзогенными гонадотропинами рост фолликулов в лютеиновую и позднюю фолликулярную фазы, что послужило базисом для формирования инновационных протоколов, направленных на получение ооцитов независимо от дня цикла: random-start протоколов и протоколов двойной стимуляции (DuoStim) [1—4]. Данные о сопоставимой эффективности классического протокола с антГнРГ в фолликулярную фазу цикла и протокола randomstart были получены в большинстве исследований, изучающих этот вопрос [1, 5].
Согласно клиническим рекомендациям, приоритетную ценность протоколы random-start имеют в тактике ведения онкологических пациентов, направленной на процедуру предварительного сохранения и криоконсервации репродуктивного материала.
Для непосредственной индукции множественного роста фолликулов в программах ЭКО рекомендовано использование препаратов гонадотропинов. При выборе конкретного препарата очень важно учитывать, что на исходы применения гонадотропина могут влиять особенности не только самого препарата, но и гонадотропин-зависимых сигнальных систем клеток-мишеней в организме конкретного человека.
Разнообразие препаратов-фоллитропинов увеличивается, а значит, появляется потенциальная возможность индивидуального подбора препарата в зависимости от особенностей организма. Следует подчеркнуть, что кломифена цитрат, а также появившиеся позднее ингибиторы ароматазы ни в прошлом, ни в настоящем не рассматривались исследователями в качестве замены препаратам гонадотропинов в программах ЭКО. Это объясняется тем, что непрямые индукторы из-за их относительно слабого стимулирующего влияния на яичники индуцируют контролируемый монофолликулярный ответ, что обуславливает целесообразность их применения в программах индукции овуляции в сочетании или без искусственной инсеминации спермой мужа или донора, а не в программах ЭКО [6].
На протяжении длительного периода времени, начиная с ранних этапов изучения возможности проведения овариальной стимуляции, единственным масштабным способом получения гонадотропинов для клинической практики была экстракция из тканей, органов или биологических жидкостей. На рассвете репродуктивной медицины гонадотропины получали из сыворотки беременных женщин и кобыл, впоследствии были разработаны способы получения индукторов из экстрактов гипофиза свиней, овец и гипофиза погибших людей.
В 1949 г. Pietro Donini предложил технику получения гликопротеинов из мочи. Но начиная с 1978 г., с момента получения первого рекомбинантного препарата — инсулина, а также после изоляция генов, кодирующих общую для молекул фолликулостимулирующего гормона (ФСГ), лютеинизирующего гормона (ЛГ), хорионического гонадотропина человека (ХГЧ) альфа-субъединицу и специфическую бета-субъединицу, появилась возможность использования рекомбинантной технологии для производства гонадотропных препаратов.
Процесс получения рекомбинантного ФСГ человека (рчФСГ) по сей день совершенствуется, от этапа получения экспрессионных векторов до разработки новых клеточных линий для их синтеза.
На рынке представлены четыре оригинальных препарата рчФСГ: фоллитропин альфа, фоллитропин бета и фоллитропин дельта, а также гипергликозилированный препарат фоллитропина альфа длительного действия. Первые два препарата получают из клеток яичников китайского хомячка (клеточная линия CHO (Chinese Hamster Ovary)), фоллитропин дельта получают в фетальных клетках сетчатки человека.
На этапе доклинических испытаний находится препарат фоллитропин эпсилон, полученный в миелоидных клетках человека.
Фоллитропин дельта, полученный в клетках линии Per.C6, был зарегистрирован в 2016 г. Особенностью данного препарата является наличие а-2,6-сиалированных гликанов, помимо а-2,3-сиалированных, а также наличие большего количества три- и тетра-сиалированных N-гликанов по сравнению с другими препаратами. Данная структурная особенность обусловлена отсутствием у клеточных линий СНО активного фермента а-2,6-сиалилтрансферазы. Это подтверждает невозможность применения животных в качестве модельных систем для оценки биологической активности фоллитропинов, полученных с использованием клеточных линий человека, и такие препараты могут быть дозированы только по массе (в мкг). Кроме того, фармакологические различия между фоллитропином дельта и фоллитропином альфа позволяют предположить, что эти препараты не могут быть рутинно заменены друг другом в клинической практике, например, в ходе одного или нескольких последовательных протоколов ВРТ.
В соответствии с отечественными, а также международными клиническими рекомендациями с целью овариальной стимуляции в программах ВРТ пациентке рекомендовано назначать как рекомбинантные гонадотропины (фоллитропин-альфа, корифоллитропин-альфа, фоллитропин-альфа + лутропин-альфа, фоллитропин-бета, урофоллитропин, фоллитропин-дельта), так и менотропины (АТХ G03GA02 — гонадотропины менопаузальные).
Коммерческие гонадотропины в России:
- высокоочищенные человеческие менопаузальные гонадотропины (ЧМГ): Мериоферт, Менопур (75 МЕ ФСГ +75 МЕ ЛГ);
- мультидозовая форма ЧМГ: Менопур Мультидоза (1200 МЕ и 600 МЕ);
- неочищенные ЧМГ: Меногон (75 МЕ ФСГ +75МЕ ЛГ);
- рекомбинантные гонадотропины для ежедневного использования: Пурегон (100 МЕ), Гонал-Ф (75 МЕ);
- рекомбинантные комбинированные препараты: Перговерис (150 МЕ ФСГ + 75 МЕ ЛГ);
- рекомбинантные гонадотропины шприц-ручка ФСГ:
- Пурегон, Гонал-Ф, Рековелль;
- рчФСГ пролонгированного действия: Элонва (150 мкг).
Необходимо также отметить существование биосимиляров — продуктов не идентичных, но аналогичных по качеству, безопасности и эффективности соответствующему им референтному препарату.
Референтный препарат — рекомбинантный фоллитропин-альфа «Гонал-Ф» (Merck Serono) используется в клинической практике с 1995 г.
Сегодня известны всего три биосимиляра, из них в России зарегистрирован лишь один:
- Бемфола (Gedeon Richter PLc. Венгрия), одобрен в 2013 г.;
- Овалип (Theramex Ирландия), одобрен в 2014 г.;
- Примапур (IVF Farma, Россия), зарегистрирован в 2019 г.
Анализ ассортиментной доступности показал, что 30% зарегистрированных ЛП представлены в форме лиофилизата для приготовления раствора для внутримышечного и подкожного введения. Треть ЛП имеют форму выпуска раствора для подкожного введения, 14% — раствора для внутримышечного и подкожного введения. Все перечисленные в данном алгоритме ЛП включены в клинические рекомендации по ведению пациенток с женским бесплодием [7, 8].
Не выявлено различий в частоте наступления беременности, осложнений и исходов беременности при использовании рекомбинантных и менопаузальных гонадотропинов для стимуляции яичников в программах ВРТ в общей популяции пациентов.
Так, по данным метаанализов, включавших 12 рандомизированных исследований, в неселективной популяции пациенток программ ЭКО применение комбинации рФСГ+рЛГ вместо монотерапии рФСГ в протоколах с десенситизацией аденогипофиза не обеспечивало достоверного улучшения результатов лечения [6, 9—11]. Следует отметить, что аналогичные данные приводились и в более ранних исследованиях [12-15].
Другие исследователи, однако, не соглашаются с такой оценкой практической значимости препаратов рЛГ и, наоборот, высказывают мнение о том, что добавление рЛГ к рФСГ при стимуляции яичников показано всем женщинам [16-18].
При этом необходимо отметить, что в соответствии с данными метаанализа, в котором проводилась сравнительная оценка эффективности применения гонадотропинов (рЛГ в сравнении с рФСГ+рЛГ) у пациенток в ходе проведения программ ЭКО, обнаружено, что среди пациенток с субоптимальным ответом получающие рФСГ+рЛГ достигали лучшей эффективности по частоте живорождения в сравнении с рФСГ [19].
Полученные исследователями данные позволили сформировать критерии необходимости добавления ЛГ-содержащих препаратов в протоколах КОС:
- при гипоталамо-гипофизарной недостаточности;
- в старшем репродуктивном возрасте;
- при субоптимальном ответе яичников на ФСГ в предыдущих протоколах.
Стартовая доза гонадотропинов определяется индивидуально на основе возраста, ИМТ и показателей овариального резерва пациентки. У пациенток с низким овариальным резервом увеличение стандартной дозы гонадотропинов не повышает ЧНБ и частоту родов живым ребенком. Стандартный режим дозирования гонадотропинов представлен в таблице 1. Индивидуализированный режим дозирования на основании уровня антимюллерова гормона (АМГ) и ИМТ для препарата фоллитропина-дельта представлен в таблице 2, для других гонадотропинов данный алгоритм не подходит.
Рандомизированное мультицентровое слепое контролируемое исследование GRAPE (2017—2021) выполнено с целью сравнения эффективности индивидуального режима дозирования фоллитропина-дельта и стандартного приема фоллитропина-альфа у пациенток в программах ЭКО. Исследование было выполнено в клиниках Китая, Южной Кореи, Тайваня и Вьетнама. Результаты по частоте развивающейся беременности в группе фоллитропина-дельта сопоставимы с группой фоллитропина-альфа (31,3% vs 25,7%). По частоте живорождения индивидуальный режим дозирования фоллитропина-дельта показал преимущество (31,3% vs 24,7%). Полученные данные коррелируют с результатами предыдущих исследований ESTER-1 и STORK, которые показывают эффективность индивидуального расчета дозировки фоллитропина у пациентов с риском СГЯ. В ходе исследований ранняя степень СГЯ без обращения за медицинской помощью выявлена у 4,0% в группе приема фоллитропина-дельта и 6,5% — в группе приема фоллитропина-альфа; с превентивными мерами — 5,0 и 9,6% соответственно [20].

У пациенток со сниженным овариальным резервом при овариальной стимуляции в программах ВРТ не рекомендовано увеличивать дозу гонадотропинов (фоллитропин-альфа, корифоллитропин-альфа, фолли- тропин-альфа + лутропин-альфа, фоллитропин-бета, урофоллитропин, фоллитропин-дельта, менотропи- ны) более 300 МЕ, так как, согласно проведенным клиническим исследованиям, увеличение суточной и суммарной дозы затраченных гонадотропинов в циклах ЭКО не приводит к увеличению ЧНБ [21].
Единственный одобренный на данный момент рчФСГ пролонгированного действия (корифоллитро- пин-альфа, торговое название Elonva, Merck) разработан путем добавления карбоксотерминального пептида в-субъединицы ХГЧ к в-субъединице чФСГ, который включает 4 дополнительных сайта гликозилирования. Данная модификация увеличивает период полувыведения рчФСГ без влияния на сборку с а-субъединицей, секрецию и т.д. [22]. Доза препарата зависит от массы тела и возраста женщины. Однократное введение препарата в дозе 100 мкг рекомендуется у женщин с массой тела <60 кг в возрасте <36 лет.

Однократное введение препарата в дозе 150 мкг рекомендуется у женщин:
- с массой тела >60 кг независимо от возраста;
- с массой тела >50 кг и старше 36 лет.
Схема овариальной стимуляции с применением препарата корифоллитропина-альфа представлена на рисунке 4.



